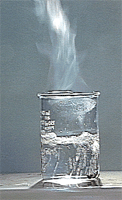The Properties of Matter
Each kind of matter possesses a number of properties by which it can be identified.
In Section 1.2A , we listed some of the properties by which the pure substance
baking soda can be identified. These properties fall into two large categories
(1) physical properties, those that can be observed without changing the composition
of the sample, and (2) chemical properties, those whose observation involves
a change in composition.
Baking soda dissolves readily in water. If water is evaporated from a solution
of baking soda, the baking soda is recovered unchanged; thus, solubility is
a physical property. The decomposition of baking soda on heating is a chemical
property. You can observe the decomposition of baking soda, but, after you make
this observation, you no longer have baking soda. Instead you have carbon dioxide,
water, and sodium carbonate. A physical change alters only physical properties,
such as size and shape. A chemical change alters chemical properties, such as
composition (see Figure 1.3).

|
|
|
|
Physical Change
|
Chemical Change
|
| FIGURE 1.3
Physical and chemical properties of matter. Breaking a stick physically
changes its size but not its composition. Burning wood changes it chemically,
turning it into other substances. |
This discussion of properties points to another difference between pure substances and mixtures. A mixture can be separated into its components by differences in their physical properties. A mixture of salt and sand can be separated because salt dissolves in water but sand does not. If we add water to a salt-sand mixture, the salt will dissolve, leaving the sand at the bottom of the container. If we pour off the water, the sand will remain. If we boil off the water from the salt solution, we will get the salt by itself. We have separated the two components of the mixture by a difference in their ability to dissolve in water. Solubility is a physical property.

Pure substances,
on the other hand, can be separated into their components only by chemical changes.
When added to water, the pure substance sodium bicarbonate does not separate into
sodium, hydrogen, carbon, and oxygen, although these components of sodium bicarbonate
differ greatly in their solubilities in water.
One of the important physical properties of a substance is its
physical state
at room temperature. The three physical states of matter are
solid,
liquid and
gas.
Most kinds of matter can exist in all three states. You are familiar with water as a solid (ice), a liquid, and a gas (steam) (Figure 1.4). You have seen wax as a solid at room temperature and a liquid when heated. You have probably seen carbon dioxide as a solid (dry ice) and been aware of it as a colorless gas at higher temperatures. The temperatures at which a given kind of matter changes from a solid to a liquid (its
melting point)
or from a liquid to a gas (its
boiling point)
are physical properties. For example, the melting point of ice (0°C) and the boiling point of water (100°C) are physical properties of the substance water.
|
|
 |
|
 |
|
|
Solid Water (ice)
|
|
Liquid Water
|
|
Gaseous Water (steam)
|
| FIGURE 1.4
The three physical states of water: ice (solid), water (liquid), and steam
(gas). |
Like pure substances, mixtures can exist in the three physical states of solid,
liquid, and gas. Air is a gaseous mixture of approximately 78% nitrogen, 21%
oxygen, and varying percentages of several other gases. Rubbing alcohol is a
liquid mixture of approximately 70% isopropyl alcohol and 30% water. Steel is
a solid mixture of iron and other pure substances.

Back Home
Next