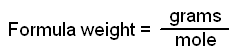
As we have observed, atoms are very small--too small to be weighed or counted individually. Nevertheless, we often need to know how many atoms (or molecules, or electrons, and so on) a sample contains. To solve this dilemma, we use a counting unit called Avogadro's number, named after the Italian scientist Amedeo Avogadro (1776-1856):
Avogadro's number = 6.02 X 1023
Just as an amount of 12 is described by the term dozen, Avogadro's number is described by the term mole. A dozen eggs is 12 eggs; a mole of atoms is 6.02 X 1023 atoms. Avogadro's number can be used to count anything. You could have a mole of apples or a mole of Ping-Pong balls. You can get some idea of the magnitude of Avogadro's number by considering that a mole of Ping-Pong balls would cover the surface of the Earth with a layer approximately 60 miles thick. Avogadro's number is shown here to three significant figures, which is the degree of accuracy usually required in calculations. Actually, the number of items in a mole has been determined to six or more significant figures, the exact number depending on the method by which the number was determined.
One mole of any substance contains 6.02 X 1023 units of that substance. Equally important is the fact that one mole of a substance has a mass in grams numerically equal to the formula weight of that substance. Thus, one mole of an element has a mass in grams equal to the atomic weight of that element and contains 6.02 X 1023 atoms of the element. For those elements that do not occur as single atoms - that is, the diatomic gases, sulfur, and phosphorus - it is important to be certain that you specify what you are talking about. One mole of atoms of oxygen has a mass of 16 g, as 16 is the atomic weight of oxygen, and contains 6.02 X 1023 atoms of oxygen. One mole of oxygen gas, which has the formula O2, has a mass of 32 g and contains 6.02 X 1023 molecules of oxygen but 12.04 X 1023 (2 X 6.02 X 1023) atoms, because each molecule of oxygen contains two oxygen atoms.
These definitions allow a new definition of atomic weight: The atomic weight of an element is the mass in grams of one mole of naturally occurring atoms of that element.
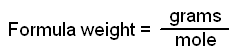
Using these relationships, we can calculate the number of atoms in a given mass of an element or the mass of a given number of atoms.