Review Questions Chapter 4
1 Which particle has the smallest mass?
2. Which particle carries no charge?
3. Which particles are found in the nucleus of an atom?
4. Uranium exists as many isotopes, one of which is![]() . Which of the following might also be isotopes of uranium?
. Which of the following might also be isotopes of uranium?
5. Rutherford's experiment is diagrammed below. Which path is followed by most of the alpha particles that hit the gold foil?
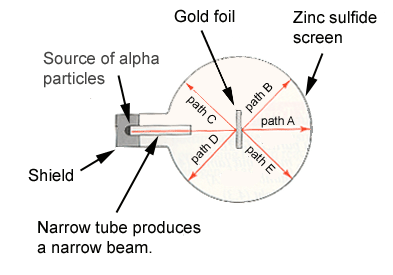
6. Why is Rutherford's gold-foil experiment so important in the history of atomic structure?
7. The atomic weight of galium is 69.72. It occurs as gallium-69 and gallium-71. What is the approximate percent of gallium-69 in naturally occurring gallium?
8. A sample of iron weighs 6.93 grams. How many moles of iron are contained in this sample?
9. What is the mass of 1.55x10-2 mol copper?
10. A sample of lead contains 3.01 x 1021 atoms. A sample of another metal contains the same number of atoms and weighs 1.01 g. What might the second metal be?