The shells are numbered in order of increasing energy.
n is called the principal quantum number.
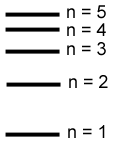
|
An atom contains protons and neutrons in the nucleus surrounded by electrons. |
|
The energy of an electron depends on its location with respect to the nucleus. |
|
Because the energy is quantized the electrons can only occupy specific regions called shells. |
|
The shells are numbered in order of increasing energy. n is called the principal quantum number. |
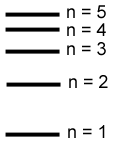 |
The energy levels in an atom are similar to the rungs of a ladder, but they get closer together as they get farther from the nucleus. |



